Covaxin for kids: Trial results may be out by September, says AIIMS chief
For Printing Download Epaper from files section from bottom of this page
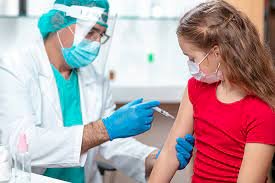
New Delhi : Bharat Biotech's Covaxin Covid-19 vaccine trials for children are currently underway and its results are likely to be out by September, said DrRandeepGuleria, the director of the All India Institute of Medical Sciences (AIIMS) in New Delhi. Last month, the AIIMS director told a Delhi-based news organisation that Covaxin shots might be available for children by September.
The vaccines for children should come out now because trials in India are already there in the vaccines which are available in India, Bharat Biotech trial is in the final phase by September, we will have the data," news agency ANI quoted DrGuleria as saying on Friday.
Reports said on Monday that the Hyderabad-based pharmaceutical company will administer its indigenous anti-Covid shot to children in the age group of 2-6 as part of its vaccination trials for kids.
In view of a probable third wave of the coronavirus pandemic, vaccination trials on children have been underway in the country. Besides Covaxin, Gujarat-based ZydusCadila is also testing its anti-Covid shot for children.
Vaccine trials for kids are conducted by dividing them into different categories on the basis of their age, with 175 participants from each age group included. Once every participant has been injected with a second dose, an interim report is expected by the end of August. On the basis of this interim trial report, a decision will be taken if the vaccine is safe to be used on children.
Covaxin, along with Ahmedabad-based ZydusCadila's three-dose shot, ZyCov-D, are the two vaccines that experts believe will be cleared for children in the near future. While Covaxin is already being used in the nationwide vaccination drive, ZydusCadila on July 1 applied for emergency use authorisation (EUA) of its vaccine candidate for those aged 12 and above.
"ZydusCadila vaccine has also included children and their data is already there. They have already applied for the emergency use authorisation," Guleria said.
Government officials, however, have repeatedly said that "more data" is required before an anti-Covid vaccine can be made available for children.
India has administered as many as 42.78 crore vaccines so far, as of 9am on Saturday. Of these shots, more than 40% have been provided to people in the age group of 18-44 years, 34.1% have been administered to 45-60 year-olds, and 25.4% to those above 60 years of age.

 Active Times
Active Times 

















